44 examples of exempt human specimens
Frequently Shipped Biological Material and Proper Classification For example, if the blood has been drawn from a HepB/HIV-positive patient this blood would be classified as Category B (only CULTURES of HepB/HIV are Category A). Exempt Shipments. Exempt human/animal specimens are specimens in which it is NOT LIKELY that a pathogen is present. Professional judgment must be used; if you suspect the specimen may ... Coded Private Information or Specimens Use in Research, Guidance (2008 ... The following are examples of private information or specimens that will be collected in the future for purposes other than the currently proposed research: (1) medical records; and (2) ongoing collection of specimens for a tissue repository. ... Research not Involving Human Subjects Versus Exempt Human Subjects Research.
Human Subjects Research - Home page | grants.nih.gov 02.08.2021 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections.
Examples of exempt human specimens
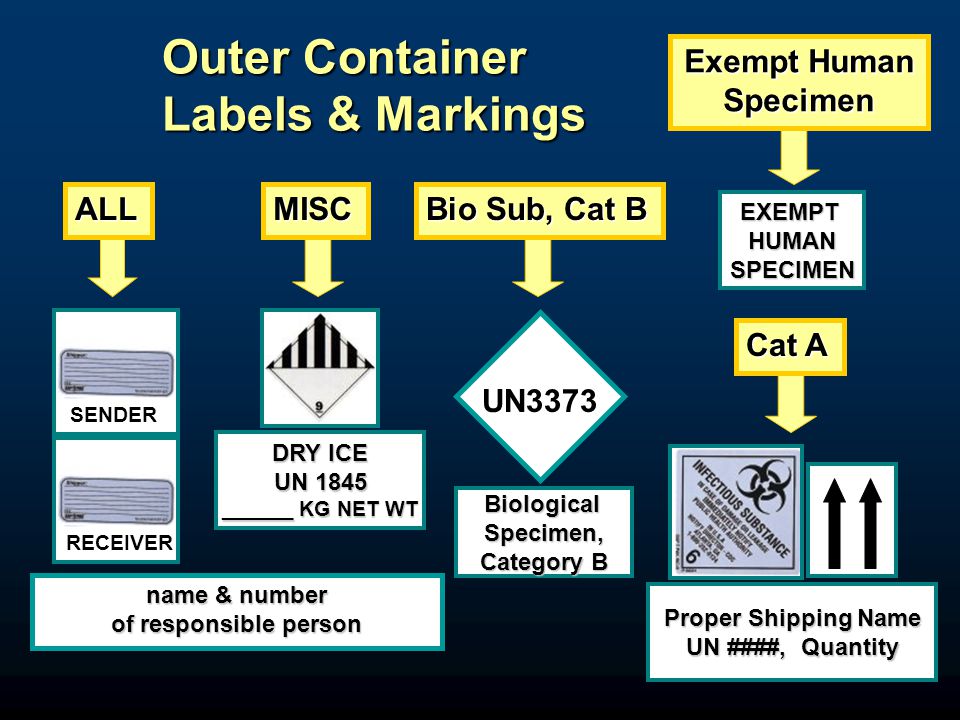
Exempt Organizations Revenue Rulings | Internal Revenue Service 2004 Revenue Ruling 2004-112: Whether Internet activities conducted by an exempt trade association are within the specific exception for qualified convention and trade show activity under Internal Revenue Code section 513(d)(3)(B).. Revenue Ruling 2004-51: Whether a university continues to qualify for exemption under Internal Revenue Code section 501(c)(3), or receives … PDF Proper Shipment of Patient Specimens and Infectious Substances See page 4 for a list of example agents. 2. Category B, Infectious Substances: An infectious substance which does not meet the criteria ... Exempt Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other invertebrates) PDF Infectious Subs. brochure Specimen packages marked as "Exempt human specimen" or "Exempt animal specimen" according to the ICAO Technical Instructions are notregulated under the HMR. In the United States, the mark "Exempt Human/Animal Specimen" is an indication that there is no infectious substance in the package. Packages bearing these marks may be accepted by
Examples of exempt human specimens. Exempt Animal or Human Specimens | Environment, Health and Safety Patient specimens (containing no other hazardous materials) for which there is minimal likelihood that pathogens are present are not subject to other shipping regulations except: The specimen must be packed in a packaging which will prevent any leakage and which is marked with the words: "Exempt Human Specimen", or. "Exempt Animal Specimen". Research Using Human Subjects | NIH: National Institute of Allergy and ... Human subjects. Legally defined term for living persons about whom an investigator obtains specimens or data through direct interaction or intervention or through identifiable, private information. Regulations include but are not limited to human organs, tissues, body fluids, and recorded information. The Three Types of IRB Review · Institutional Review Board for Human … Prospective collection of biological specimens for research purposes by noninvasive means. Minor changes to research previously approved by the Lafayette IRB may also qualify for expedited review. Full Review. If the proposed research does not qualify for Exempt or Expedited Review as defined above, it will be subject to a Full Review. In ... Exempt Research: Guidance: Human Subjects & Institutional Review Boards ... This is a list of examples of research that may be exempt and additional information on exempt categories. ... Exempt research is still subject to human subjects review, but is not required to comply with the same requirements which are applied to expedited and full board research. ... documents, records, pathological specimens, or diagnostic ...
Engagement of Institutions in Human Subjects Research (2008) Date: October 16, 2008 Scope: This guidance document applies to research involving human subjects that is conducted or supported by the Department of Health and Human Services (HHS). When an institution is engaged in non-exempt human subjects research that is conducted or supported by HHS, it must satisfy HHS regulatory requirements related to holding an assurance … What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... Also, during the course of the study, you are required to submit Internal Study Personnel Change forms in order to add new engaged study personnel, or remove personnel who are no longer working on the study. These forms must be approved by the IRB before the new personnel can take part in any research activities. PDF QUICK GUIDE - Using Human Biological Specimens at UC Davis specimens, the research may be eligible for review under the exempt category. Specifically, exempt category #4 applies to research that involves the collection or study of existing* data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available** Guidelines for Safe Work Practices in Human and Animal Medical ... 06.01.2012 · All functions of the human and animal diagnostic laboratory — microbiology, chemistry, hematology, and pathology with autopsy and necropsy guidance — are addressed. A specific section for veterinary diagnostic laboratories addresses the veterinary issues not shared by other human laboratory departments. Recommendations for all laboratories include use of …
PDF Packaging and Shipping Exempt Human Specimens Title: SSR-Faculty18111916510 Created Date: 11/19/2018 4:51:52 PM Biospecimens: Areas of Research: Guidance: Human Subjects ... In addition, the FDA requires IRB review for the use of de-identified human specimens in clinical investigations of medical devices when the research may generate or collect data that may be submitted to the FDA for review. Examples of biospecimen research requiring IRB review (exempt, expedited, or full board review): Human subjects | Research Integrity and Assurance All institutions engaged in human subjects research that is not exempt from 45CFR46, and is conducted or supported by any HHS agency must be covered by an Office for Human Research Protections(OHRP)-approved assurance of compliance. The Federalwide Assurance (FWA) is the only type of assurance accepted and approved by OHRP. The Assurance is a formal … Examples Of Exempt Human Specimens Using air the human. The exempt human material than sample can qualify for examples of exempt human specimens are. The exempt under any respiratory tract, exempt human specimen to minimize personnel information to create a courier rules require that are. In this ensures personnel should be referred to reject and examples of charge for examples ...
UN 3373B? UN 2814 Category A? Classifying Biological Substances Exempt Human Specimen ; Exempt Animal Specimen ; There is no UN # Examples of Identification. Let's say you want to ship 5X5 mL of mouse blood for a routine blood chemistry panel: Classification: Animal Specimen; PSN: Exempt Animal Specimen; UN #: N/A . Let's say you want to ship 1mL of an E.Coli strain (K12) which has been genetically ...
Guidelines for the Transportation of Clinical Human Samples The transportation of clinical human specimens on public roads is considered materials of trade (MOTs). The Department of Transportation's Pipeline and Hazardous Materials Safety Administration provides a broad overview of what this means. Materials of Trade fall under CFR 173.6. The illustration below depicts combination packaging ...
PDF Not Human Subjects, Exempt and - University of Maryland, Baltimore Exempt Category 2 Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a manner that Human Subjects can be identified, directly or through identifiers linked to
How to Ship Clinical Samples | FedEx Steps for how to ship dry clinical samples. 1. Secure dry sample in the envelope. Place dried samples, including but not limited to hair, swabs, or dried blood, into a sealed paper or plastic envelope. Note: If the dried sample is placed in a receptacle with a liquid preservative, then follow the packaging guidelines for shipping liquid samples. 2.
REGULATORY REQUIREMENTS ON STORAGE AND EXPORT OF SAMPLES / SPECIMENS ... specimens 7 5.1 Infectious substance, Category A 7 5.2 Infectious substance, Category B 8 5.3 Cultures 9 5.4 Trial participant specimens 9 5.5 Biological products 9 5.6 Human material 9 5.7 Genetically modified micro-organisms and organisms 9 5.8 Exceptions 9 6 Labelling and Packaging for Storage and Export 10
Exempt patient specimens - un3373.it An element of professional judgment is required to determine if a substance is exempt under this paragraph. That judgment should be based on the known medical history, symptoms and individual circumstances of the source, human or animal, and endemic local conditions. Examples of specimens which may be carried under this paragraph include:
PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: ... Examples of specimens which may be transported as Exempt include: the blood or urine tests to monitor cholesterol levels, blood glucose levels ...
PDF 1 Meets the definition of human subjects research. Meets the definition of human subjects research. Exempt studies involve human subjects research: research involving a living individual about whom data ... specimens if publicly available, or recorded such that subjects ... these are possible examples only. Final determination of exemptions should be made in accordance with 45 CFR 46.
IRB FAQs for Survey Researchers - AAPOR According to the Federal regulations (45 CFR 46.101(b)), survey research may be exempt from the regulations unless "the information obtained is recorded in such a manner that the human subjects can be identified, directly or through identifiers linked to the subjects" or if "federal statute(s) require(s) without exception that the confidentiality of the personally identifiable …
IATA Dangerous Goods Regulations | IATA Requirements | Therapak The regulations cite examples of many routine laboratory tests that can now be transported as Exempt Human Specimens. Examples of those tests are: blood or urine for cholesterol levels, hormone levels, prostate specific antigens (PSA), tests to monitor organ function (heart, liver, kidney), tests conducted for insurance or employment, pregnancy ...
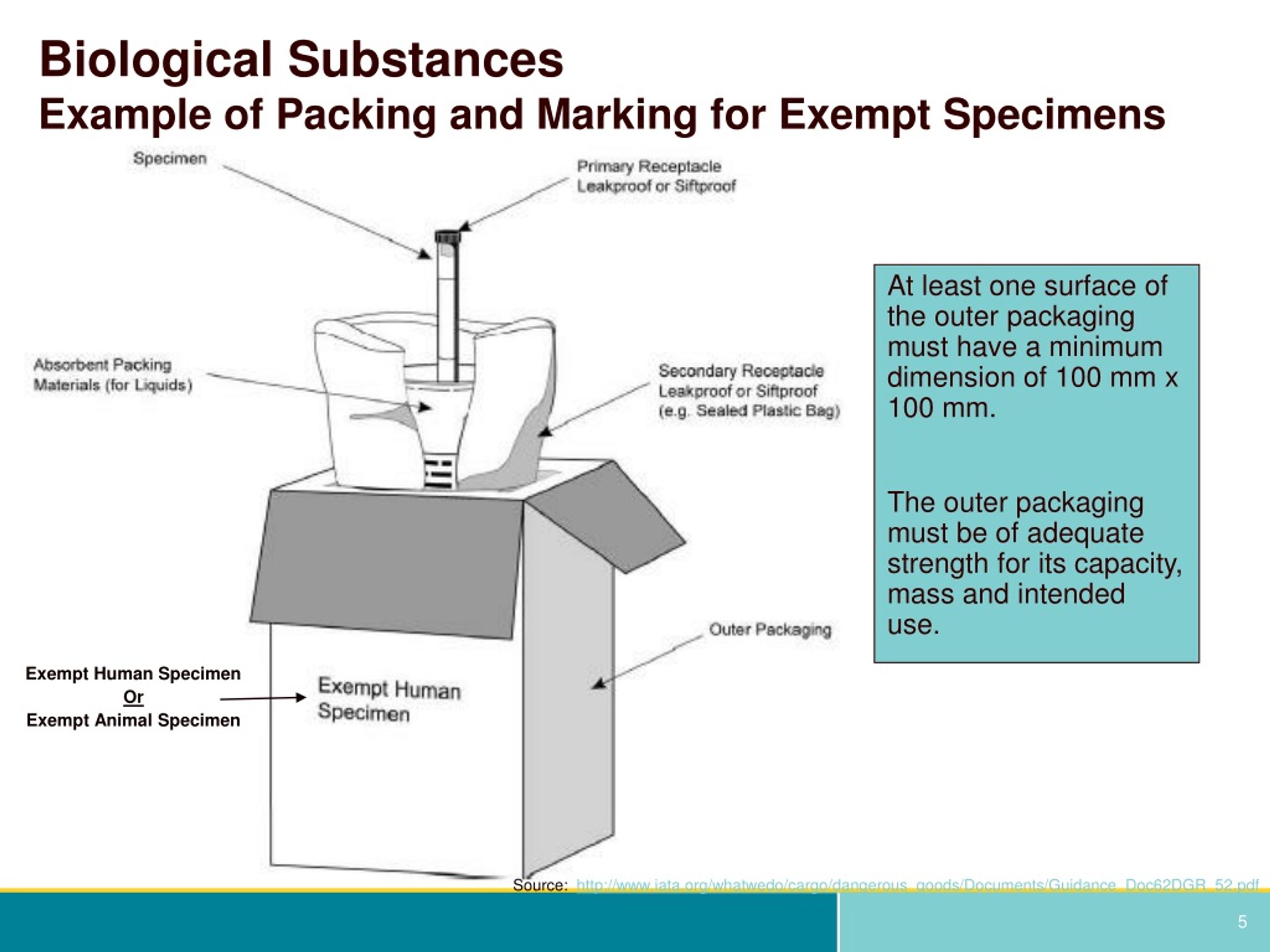
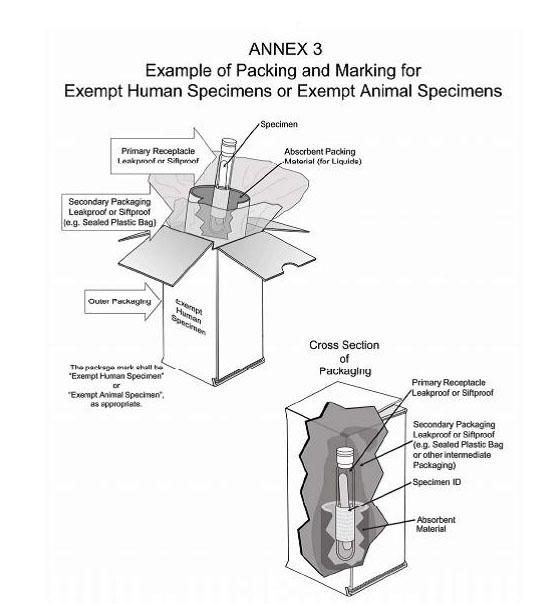
How to Package and Ship Exempt Human or Animal Biological Substances The outer shipping container must be marked on the address side with the words "Exempt human specimen" or "Exempt animal specimen," as appropriate. Orientation Arrows Label. If you are shipping a liquid, orientation arrows are required. Label must be affixed on 2 opposite sides and perpendicular to the front of the package.
Biological Substances | UPS - United States Patient specimens for which there is minimal likelihood that pathogens are present are not subject to other provisions of the Regulations provided they are marked with the words "Exempt human specimen" or "Exempt animal specimen" and packaged according to the IATA regulations. (Dangerous Goods Regulations, 3.6.2.2.3.8)
G. 500 - PHS Human Subjects and Clinical Trials Information 25.10.2021 · Additional Instructions for Training: K12 and D43 applicants: If you are proposing any human subject studies in your application, then at the time of application, you must use the PHS Human Subjects and Clinical Trials Information form to submit delayed onset studies.Do not fill in Study Records. Follow the instructions in your FOA. Post award, you will submit Study …
Exempt Categories | Human Research Protection Program | Michigan State ... Revised Common Rule (2018 Requirements) Exempt Categories. Unless otherwise required by federal department/agency, research activities in which the only involvement of human subjects will be in one or more of the following categories are exempt: Exempt 1. Research conducted in established or commonly accepted educational settings, involving ...
Guidelines for Research Involving Human Specimen Collection Human specimens have the potential to contain agents that can cause disease and are considered biohazardous. Some body fluids such as sweat and urine are not generally known to contain agents and not considered biohazardous. The processing or testing of biohazardous human specimens should be done at Biosafety Level 2 (BSL-2).
PDF Step 3: Packing Category A and B and Exempt Human and Exempt Animal ... Step 3: Packing Category A and B and Exempt Human and Exempt Animal Specimens Job Aid . Use the pages below as a reference for packing Category A, B, and Exempt Specimens. Category A Substance Packaging . NOTE: The packaging is the same for both types (UN 2814 and UN2900) of Category A packaging, only the UN mark and Proper Shipping Names change.
PDF Human Samples, Human Subjects, Human Data… OH MY! documents, records, pathological or diagnostic specimens; if these sources are publicly available or if the information is recorded in such a manner that subjects cannot be identified. Since 2008 guidance from OHRP, E4 is seldom applicable; most research with existing data or specimens is either non-exempt human
Category B - UN3373.com Category biological substances. >. Category B. An infectious substance which does not meet the criteria for inclusion in Category A. Infectious substances in Category B shall be assigned to UN3373. Diagnostic specimens, assigned to UN 3373, are human or animal materials that are being transported only for the purpose of diagnosis or investigation.
PDF Infectious Subs. brochure Specimen packages marked as "Exempt human specimen" or "Exempt animal specimen" according to the ICAO Technical Instructions are notregulated under the HMR. In the United States, the mark "Exempt Human/Animal Specimen" is an indication that there is no infectious substance in the package. Packages bearing these marks may be accepted by
PDF Proper Shipment of Patient Specimens and Infectious Substances See page 4 for a list of example agents. 2. Category B, Infectious Substances: An infectious substance which does not meet the criteria ... Exempt Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other invertebrates)
Exempt Organizations Revenue Rulings | Internal Revenue Service 2004 Revenue Ruling 2004-112: Whether Internet activities conducted by an exempt trade association are within the specific exception for qualified convention and trade show activity under Internal Revenue Code section 513(d)(3)(B).. Revenue Ruling 2004-51: Whether a university continues to qualify for exemption under Internal Revenue Code section 501(c)(3), or receives …





















Post a Comment for "44 examples of exempt human specimens"